Lithium Reaction With Water
In the case of a lithium-ion rechargeable battery the reaction proceeds like this. Combining different types of batteries can result in a reaction even if they are taped.
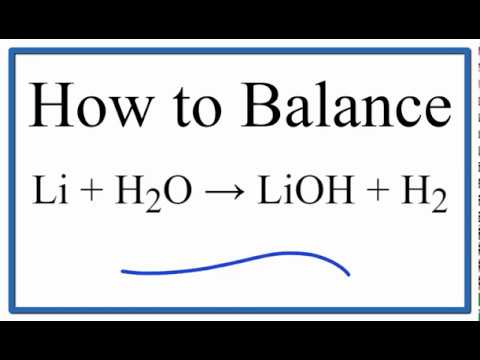
How To Balance Li H2o Lioh H2 Lithium Plus Water Youtube
The lithium ion and.

. Make a Diet Plan Most people cringe when they hear the word diet but usually its because they associate it with fad diets that involve something like eating only carrots or starving themselves. Lithium batteries are generally safe but there are a few things you should know to protect your workers and your facilities. Ignites on contact with water.
Research into fusion reactors began in the 1940s but to date no design has. To understand why lithium-ion batteries can pose a safety hazard it can be helpful to understand how they work. Flouride has also been touted as another alternative to lithium and has the potential for much greater storage capacity.
How lithium-ion batteries work. The fluorine typically reacts with hydrogen to form hydrogen fluoride HF. Lithium-sulphur batteries LSBs have one of the great potentials for serving as next generation high energy density batteries.
If you are taking lithium and also exercising regularly it is especially important that you drink extra water to compensate for lithiums side effect of dehydration. As per recent market research reports the graphene battery market including graphene battery lithium-sulphur battery and graphene supercapacitors end use industries like electronics automotive power etc the market size is projected to grow from. Heres a quick chemistry lesson.
6 dissolved in the electrolyte. The lithium ion migrates through the internal structure of the battery while the electron exits the battery and flows through whatever circuit the battery is attached to. Devices designed to harness this energy are known as fusion reactors.
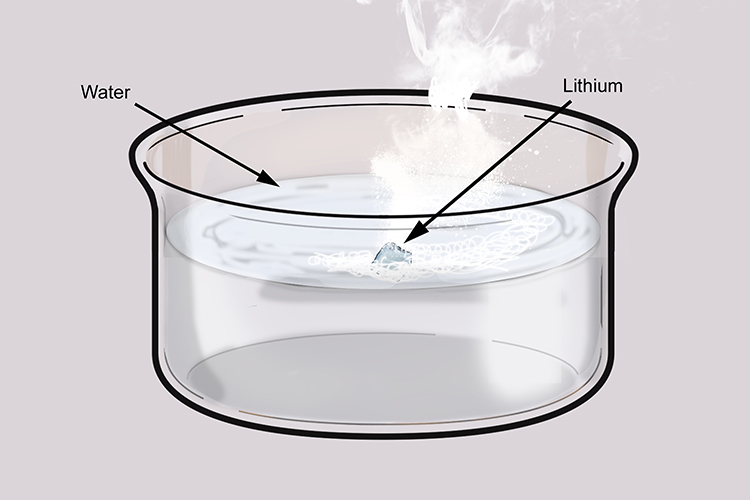
3 Lithium and all other alkali metals tend to react violently with water and thus it cannot be prepared from the aqueous solution of its salt by the normal metal displacement method. Violent reaction with water. Safety Program Emergency Manager Chuck Emnett 406 243-4504.
Lithium aluminium hydride commonly abbreviated to LAH is an inorganic compound with the chemical formula Li Al H 4It is a white solid discovered by Finholt Bond and Schlesinger in 1947. O as the by -products. Wash White Clothes by Hand.
2 It cannot be extracted from its ores by the process of electrolysis of aqueous solutions as the resultant metals will end up producing their hydroxides on immediate reaction with water. This Review details recent advances in battery chemistries and systems enabled by solid electrolytes including all-solid-state lithium-ion lithiumair lithiumsulfur and lithiumbromine. Sodium-sulphur batteries have longer lifespans than lithium-ion batteries though there are risks involved with handling both sodium infamous for its reaction with water and sulphur.
The burning reaction also tends to liberate the fluorine from the lithium salt typically LiPF. You need to place them in separate. This compound is used as a reducing agent in organic synthesis especially for the reduction of esters carboxylic acids and amidesThe solid is dangerously reactive toward water releasing.
Gives off explosive acetylene gas. A guide for safely disposing of dead batteries Lithium and lithium-ion or Li-ion batteries are commonly used to power. Paula Short paulashortumontanaedu 406 243-5806.
Violent or explosive reaction when water added. For most batteries the products typically consist of CO. As the battery discharges one lithium atom at the negative electrode splits into a lithium ion and an electron.
Fusion power is a proposed form of power generation that would generate electricity by using heat from nuclear fusion reactionsIn a fusion process two lighter atomic nuclei combine to form a heavier nucleus while releasing energy.

How Does Lithium Metal React With Water Quora

Information On Alkali Metals Stanford Environmental Health Safety

0 Response to "Lithium Reaction With Water"
Post a Comment